Model informed Drug Development
12th & 13th June 2019, National and Kapodistrian University of Athens, Athens
PEARRL is an EU research and training network focused on exploring new ways to streamline drug product development to facilitate earlier access to innovative new medicines. Regulatory initiatives such as “Earlier Access to Medicines” (MHRA, 2014) and “Adaptive Licensing”(EMA, 2014) highlight the recent increasing role regulatory agencies are playing in supporting this area[1]. A key objective of PEARRL is to increase awareness of advances in Regulatory Science (i.e. the science of “developing new tools, standards and approaches to assess the safety, efficacy, quality and performance of regulated products”).
Building on the success of the 2017 and 2018 symposium, PEARRL will host a Symposium on Model Informed Drug Development at the National and Kapodistrian University of Athens, in Athens, on 12th & 13th 2019.
The theme for Day 1 of the Open Symposium is "Lead optimisation through to pre-clinical development". Day 2 of the symposium will be focused on "Clinical development through to pharmaceutical product lifecycle management".
An outline agenda for both days is provided below.
If you are interested in attending one or both days of the Symposia, you should register your interest in attending on the field below. There is no charge for registration but places are limited, so you must register by the 31st May 2019 if you wish to attend.
Building on the success of the 2017 and 2018 symposium, PEARRL will host a Symposium on Model Informed Drug Development at the National and Kapodistrian University of Athens, in Athens, on 12th & 13th 2019.
The theme for Day 1 of the Open Symposium is "Lead optimisation through to pre-clinical development". Day 2 of the symposium will be focused on "Clinical development through to pharmaceutical product lifecycle management".
An outline agenda for both days is provided below.
If you are interested in attending one or both days of the Symposia, you should register your interest in attending on the field below. There is no charge for registration but places are limited, so you must register by the 31st May 2019 if you wish to attend.
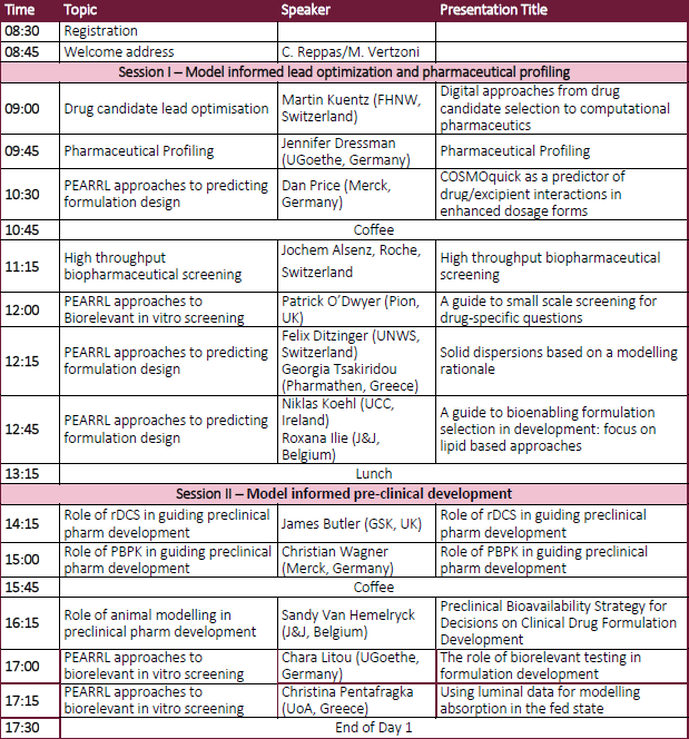
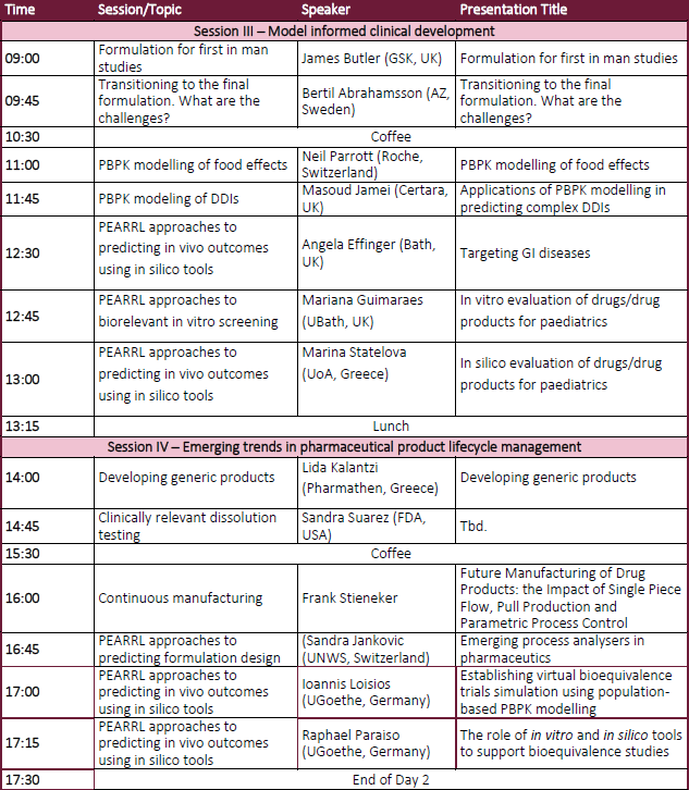
Day 1 - Agenda (provisional)Day 2 - Agenda (provisional)Invited Speakers (Provisional list)
About PEARRL PEARRL is a European Training Network (ETN) funded under the Horizon 2020 Marie Sklodowska-Curie actions. It brings together European Pharma industry, academia and regulatory agency partners in a multisectorial team to deliver a unique research and training programme for 15 Early Stage Researchers (ESR). The main research objectives of the PEARRL research programme are to deliver novel bio-enabling formulations and new biopharmaceutics tools to predict their in vivo performance as a means to improve efficiency and cost-competitiveness in drug development, thus facilitating earlier access of patients to “breakthrough therapies”. PEARRL will train 15 Early Stage Researchers (ESR) who can develop such new bio-enabling formulations (“better drugs”), biorelevant and in silico methods to predict formulation performance in vivo (“streamlined development”) and serve as communication bridgers between research and regulatory science (“accelerated approval”), thus bringing Pharma and regulatory objectives to fruition. |
Registration for the PEARRL Symposium is open
For Non-PEARRLers to attend the 2-day open symposium they must be registered.
Certificate of attendance will not be provided to attendees. Registration will close 31st May, 2019, or when the upper limit of registrations is reached, i.e. twenty (20). Please register to the PEARRL Symposium 2019 via the following registration form |