The first Annual PEARRLS of Wisdom Week in Cork, Ireland kicked off with a Symposium on Bioenabling Formulations for Oral Drug Delivery, featuring lectures from Consortium Members as well as guest speakers.
The Principal Investigator of PEARRL, Brendan Griffin, and the Work Package 1 Leader, Martin Kuentz, welcomed the attendees to Cork and to the PEARRLS of Wisdom Week and briefly outlined the Programme for the day. This was followed by an overview of why bioenabling formulations are often needed for the new chemical entities (NCE) being developed for oral delivery, entitled “Pimp My Drug”. Professor Jennifer Dressman (Goethe University) presented data showing the rise in % of poorly soluble NCE and explained development goals in terms of a shift from a class 2/4 NCE to a formulation with essentially class 1/3 behaviour. She then gave an overview of the various pharmaceutical approaches to achieve these goals, including a discussion pf potential advantages and disadvantages of each strategy, to set the stage for the following presentations.
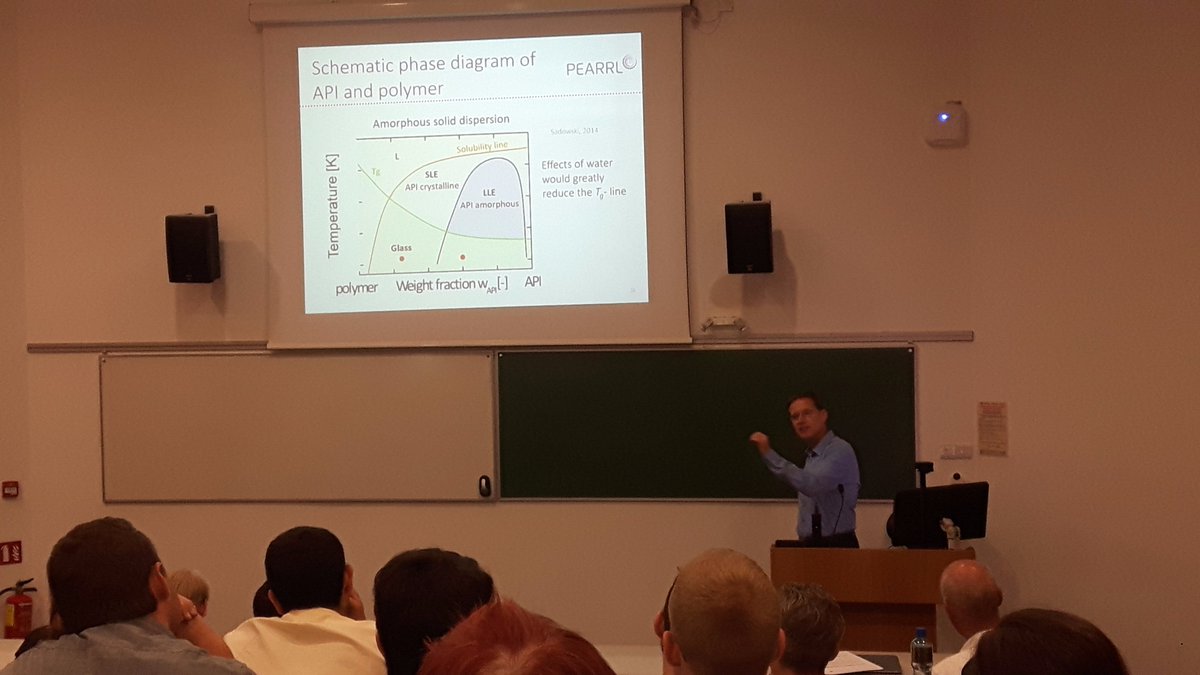
In his presentation, “Life as a Matter of Fat”, Dr. Brendan Griffin (UCC) explained how lipid based formulations exploit the benefits of a postprandial environment. He highlighted the advantages of SEDDS, which form microemulsions with a very consistent particle size of about 50-140nm and extremely low interfacial tension (<0,1mN/m), indicating that these actually behave like swollen micelles than a traditional emulsion. The mechanism(s) by which SEDDS can increase bioavailability are thought to be via break up of micelles, releasing the drug to the intestinal membrane (primary), increasing the membrane fluidity and, for extremely lipophilic drugs, facilitating lymphatic transport. The next presentation was “Nuts and Bolts of Solid Dispersions”, in which Dr. Martin Kuentz (FHNW) focused on solvent based solid dispersions. Dr. Kuentz explained important thermodynamic transitions essential to successful formation of a solid dispersion. As the goal is to form a glassy solid solution that is stable over time, it is important to limit even ß-relaxation. This requires a solid dispersion that has a glass transition temperature at least 10° higher than the storage conditions. It is important to remember that water acts as a plasticizer to many of these systems, so water uptake can jeopardize the stability of the dispersion. Another problem that was discussed was the low density of the product and it was mentioned that drying under slow, cool conditions leads to a higher density in a spray-dried product than rapid drying at high temperatures.
In his presentation, “Life as a Matter of Fat”, Dr. Brendan Griffin (UCC) explained how lipid based formulations exploit the benefits of a postprandial environment. He highlighted the advantages of SEDDS, which form microemulsions with a very consistent particle size of about 50-140nm and extremely low interfacial tension (<0,1mN/m), indicating that these actually behave like swollen micelles than a traditional emulsion. The mechanism(s) by which SEDDS can increase bioavailability are thought to be via break up of micelles, releasing the drug to the intestinal membrane (primary), increasing the membrane fluidity and, for extremely lipophilic drugs, facilitating lymphatic transport. The next presentation was “Nuts and Bolts of Solid Dispersions”, in which Dr. Martin Kuentz (FHNW) focused on solvent based solid dispersions. Dr. Kuentz explained important thermodynamic transitions essential to successful formation of a solid dispersion. As the goal is to form a glassy solid solution that is stable over time, it is important to limit even ß-relaxation. This requires a solid dispersion that has a glass transition temperature at least 10° higher than the storage conditions. It is important to remember that water acts as a plasticizer to many of these systems, so water uptake can jeopardize the stability of the dispersion. Another problem that was discussed was the low density of the product and it was mentioned that drying under slow, cool conditions leads to a higher density in a spray-dried product than rapid drying at high temperatures.
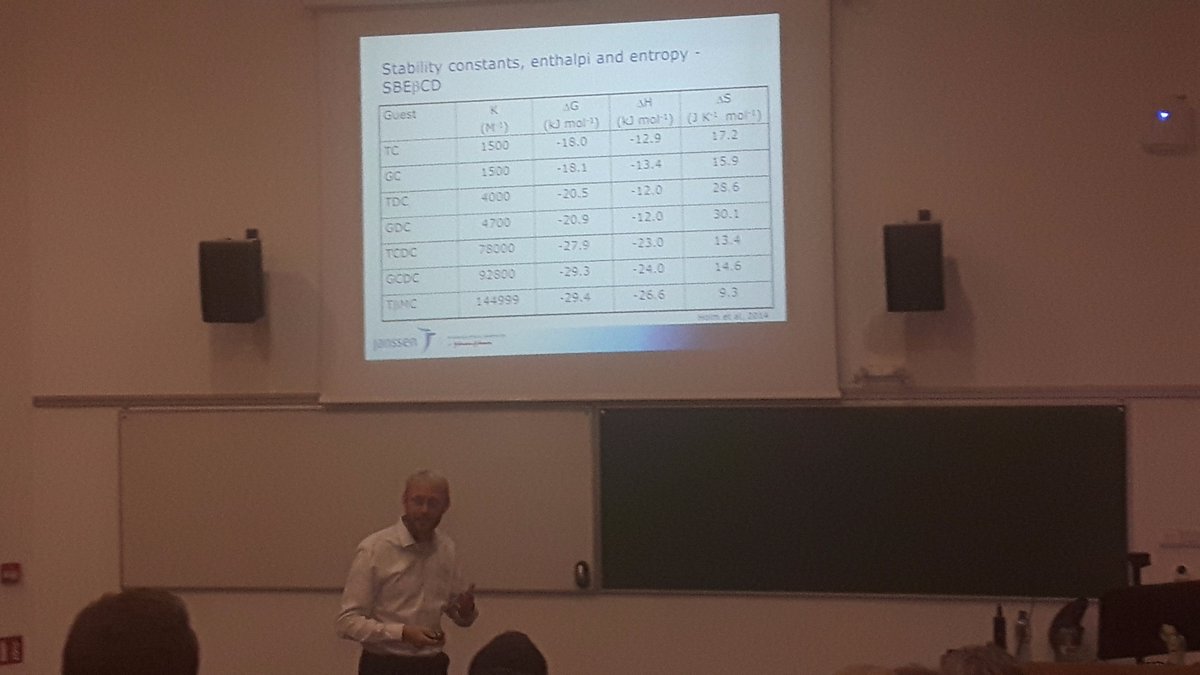
Dr. Albina Crean (UCC) talked about adsorption onto mesoporous silica as an alternative way to generate faster drug release and thus better drug absorption (“Adsorption for Absorption?”). There are various ways to load the drug onto the silica, including solvent impregnation (dropwise or slurry) and loading from supercritical fluids like carbon dioxide. The advantage of the latter is that no solvent recovery is required post-processing, but scale-up might constitute a challenge in terms of equipment availability for many pharmaceutical companies. The fourth presentation was given by Dr. Rene Holm (Janssen NV) on the subject of complexation with cyclodextrins (“Ring-a-Ling: Cyclodextrins and the Art of Inclusion”). Cyclodextrin formulations are available on the market for many routes of administration including oral dosage forms. There seems to be some advantage in terms of bioavailability in administering the drug as a CD solution compared to a simple physical mixture in a solid dosage form although even the latter strategy can produce significant increases in fraction absorbed. As NCE molecules are getting bigger, there are some issues with getting them to fit into a classical ß-CD ring. Gamma-CDs, which are larger, are not a good option as they tend to collaps due to the large ring opening. A further problem with CDs is that the CD is a large molecule, adding to the bullk of a solid, oral dosage form. This means that the drug dose should be less than 100mg in order to achieve a dosage form that is easy to swallow. Dr. Holm also emphasized the need to elucidate the precise nature of the interaction between the CD and the drug during development and illustrated the use of ROESY-NMR to do this.
Dr. Jan Möschwitzer (Advance Pharma) discussed the strategy of nanosizing the NCE to improve dissolution rate („Nanosize me! Smaller may be better for drug absorption“). Methods for nanosizing include pearl milling and high pressure homogenization and variations on these two techniques. The most suitable candidates are “brickbats” – NCE with high melting points that are not ionizable, are dosed in high amounts. NCE that are unstable at high temperature are also candidates for this strategy. The solubility of the NCE should be at most 40µg/ml, taking the surface modifiers into account. Surface modifiers can be electrostatic (e.g. anionic surfactants like SDS) and/or steric (e.g. non-ionic surfactants or polymers like HPMC E5 or PVA) in nature, whereby those that have elements of both are usually more successful in preventing re-aggregation of NCE particles. The next speaker was Justin Thian (U-Belfast) who discussed the versatility of hot melt extrusion (“The Urge to Merge: Hot melt extrusion”). Not only can this technique solve solubility issues, but also may be the wave of the future due to the possibility of continuous manufacturing and combining it online with other techniques. Like Dr. Kuentz, he stressed that solid dispersions come in many forms. Ideally, a glassy solid solution would be formed. He emphasized that it is not always necessary for the NCE to have a melting point below the processing temperature, as the NCE may be able to dissolve in the polymer.
| Dr. Albina Crean (UCC) | Dr. Rene Holm (Janssen NV) |
The last speaker of the day was Dr. Christoph Saal (Merck KGaA) with the topic of “To Salt or Not to Salt”. Points in the development chain where a salt form can be entertained are either very early in development or in life cycle management. Choice of salt partner is multifaceted but a key rule of thumb is that the difference in the pKa of the parent compound and salt partner should be at least two units. Otherwise, a co-crystal may be formed rather than a salt, leading to a different regulatory pathway under current ICH rules. Further considerations in choice of a salt partner are the toxicity and pharmacology of the partner and the physical characteristics of the salt formed e.g. melting pint, hygroscopicity, the increase in molecular weight (may be desirable for very potent drugs in terms of obtaining homogenous mixes with excipients, but undesirable due to the increase in mass load for drugs requiring high doses), and crystal habit, which can affect e.g. flow properties and filtration efficiency. Dr. Saal wrapped up his talk by noting that while the free acid or free base is usually more suitable for incorporation into enabling formulations, formation of a salt may circumvent the need to search for an enabling formulation.
Overall, the Symposium provided the ESRs, PEARRL scientists and guests with an open and honest appraisal of the various strategies to improve the absorption of poorly soluble NCEs through enabling formulations, highlighting both pros and cons of each strategy and indicating where research is still needed to optimize the implementation of these important techniques.



 RSS Feed
RSS Feed
